Periodic Trends
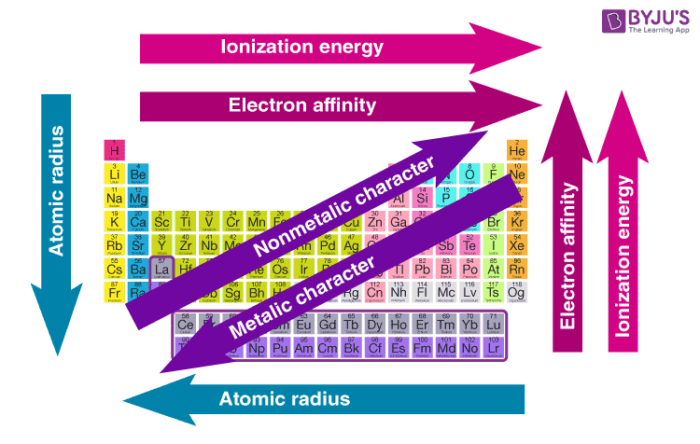
I'm pretty busy with the rest of the site, so take this Byju's diagram for now.

Plum puddings and electrons galore!
The periodic table is essentially a strategic layout of elements based on atomic number and characteristics, created by Dmitri Mendeleev.
The zigzag in the middle marks the division between nonmetals (on the right) and metals (on the left), with semimetals along the line. The farther an element is from the zigzag, the more reactive it is.
Elements react to get full valence shells for stability (noble gases have this so they don't react.)
Alkali metals. Very reactive, like to lose one electron.
Alkaline-Earth metals. Less reactive and like to lose two electrons.
Transition metals. Somewhat reactive-- they're the ones you generally think of when metals are mentioned.
Metalloids. Kinda metals but kinda not; pretty stable.
Halogens. Very reactive and are gases.
Noble gases. Unreactive gases that have full valence shells.
I'm pretty busy with the rest of the site, so take this Byju's diagram for now.

After reacting with others, atoms of elements become ions-- charged versions of their old selves. They are either positive or negative; losing electrons make you positive and gaining them makes you negative.
Isotopes are variants of atoms with differing neutron counts (they are still neutral).
Also, electrons are negative, protons are positive, and neutrons are neutral. Protons and neutrons are in the nucleus and larger than electrons, and electrons orbit around that in electron shells.
"How can I remember this?" you ask. Fear not, for I have a diagram:
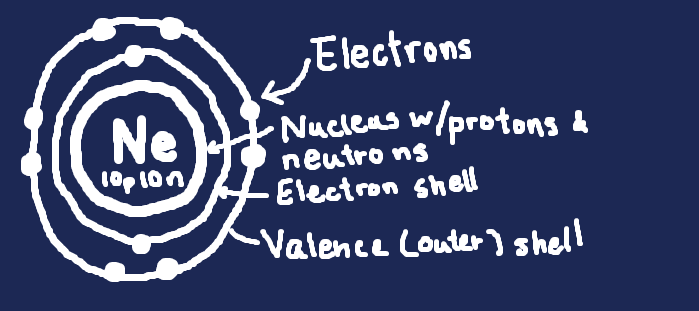
This is a Bohr diagram. Take note of how many electrons are in each shell; two in the first, then eight in the next. The next has eight too, and all other ones have sixteen. Use these to determine the valence electrons of any element!
There are two types of chemical bonds-- ionic and covalent. Ionic ones exchange electrons (between nonmetals and metals) while covalent ones share them (only nonmetals).
To name an element, we first need to know the formula. An example is H2O, the formula for water. This means there are 2 hydrogen molecules and one oxygen molecule. You know this bond is covalent because it is between two nonmetals.
Thus water would be dihydrogen monoxide.
For multivalent metals (ones with multiple ion charges), put Roman numerals in brackets after the name, like Iron (II) oxide.